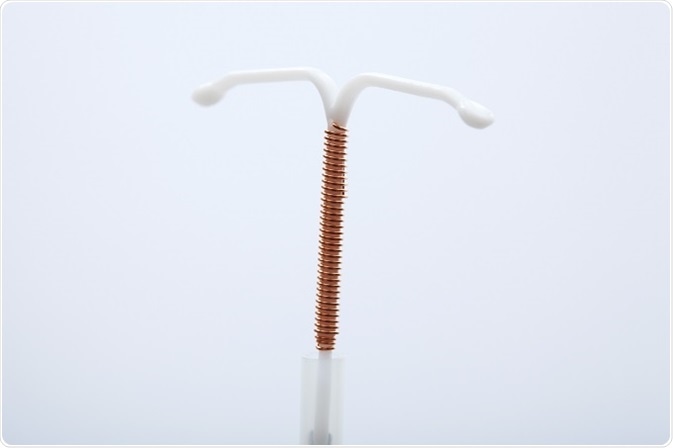
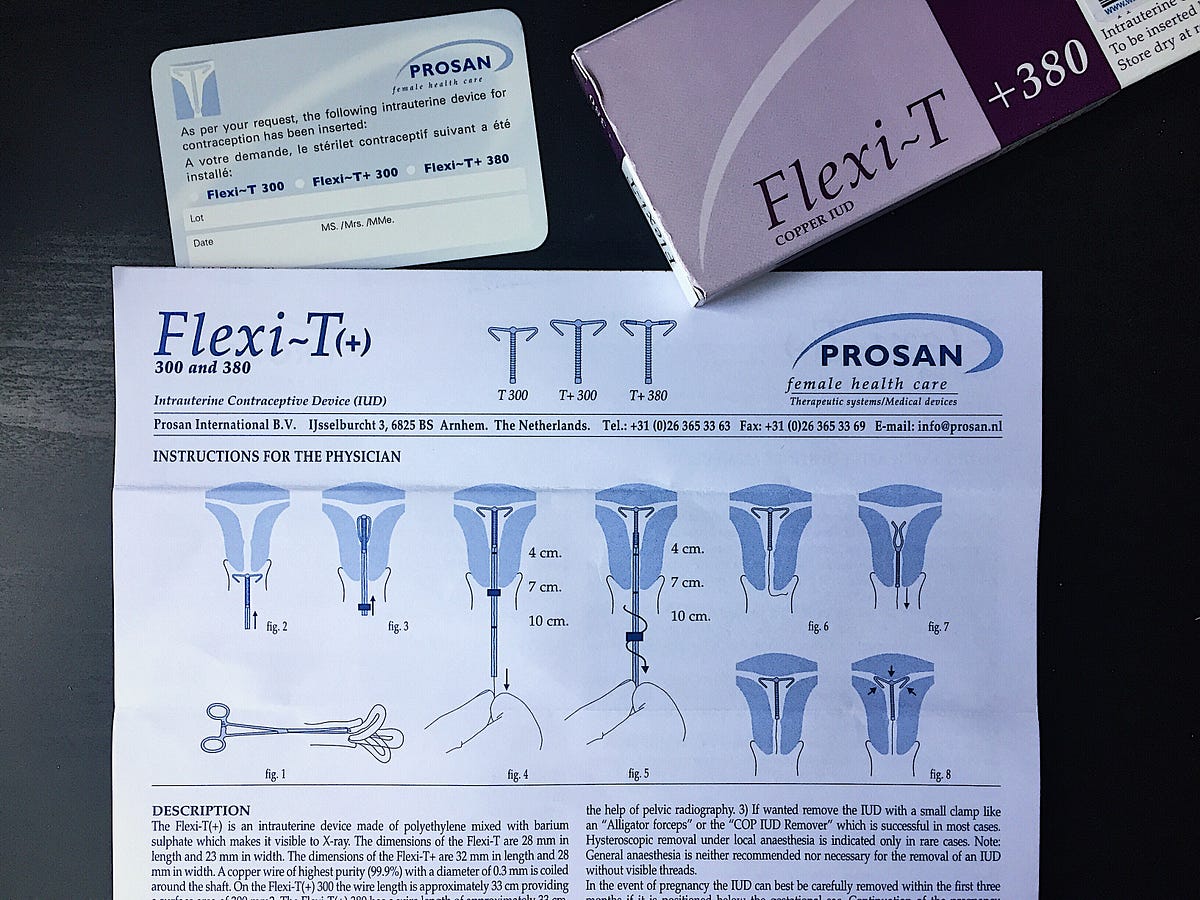
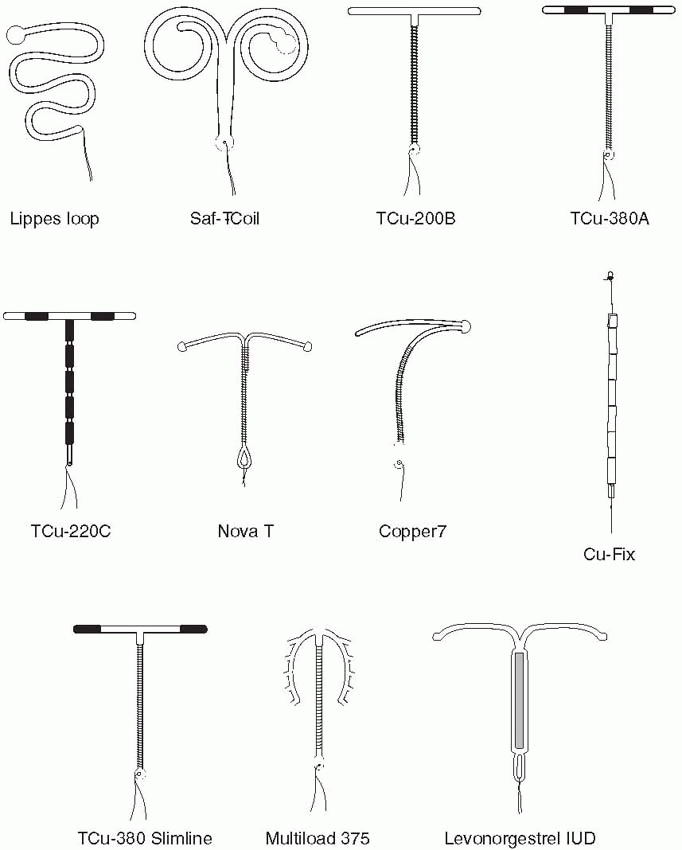
nova t copper iud sorted by
relevance
-
Related searches:
- girls selfie
- ursula corbero nude
- angie degrazia naked
- really ugly man
- who is diplo dating
- Camila Morrone nackt
- Davin Lexen nackt
- Jessica Howell nackt
- porno debby ryan
- jung und frei
- sex oglasi sa brojem telefona
- Catherine Bode nackt
- poznanstva kontakti njuskalo
- scat and piss

Admin14.08.2021
4500

Admin06.07.2021
2700

Admin21.08.2021
3105